41 examples of exempt human specimens
Shipping of Biological Samples - International / USA An example of an item description would read "Exempt Human Serum Samples for academic testing. Non-infectious, No Commercial Value". If you are typing this description, ensure that it fits in the provided area and does not get cut-off during DO NOT reference the price you are paying for the assay service on ANY documents. Exempt Research: Guidance: Human Subjects & Institutional Review Boards ... Students and Use of Education Records in Research Use of Protected Health Information (PHI) in Research This guidance provides examples of research which may be exempt and additional considerations for each available exempt category. Exempt research studies approved in 2020 were migrated to Kuali Protocols in January 2021.
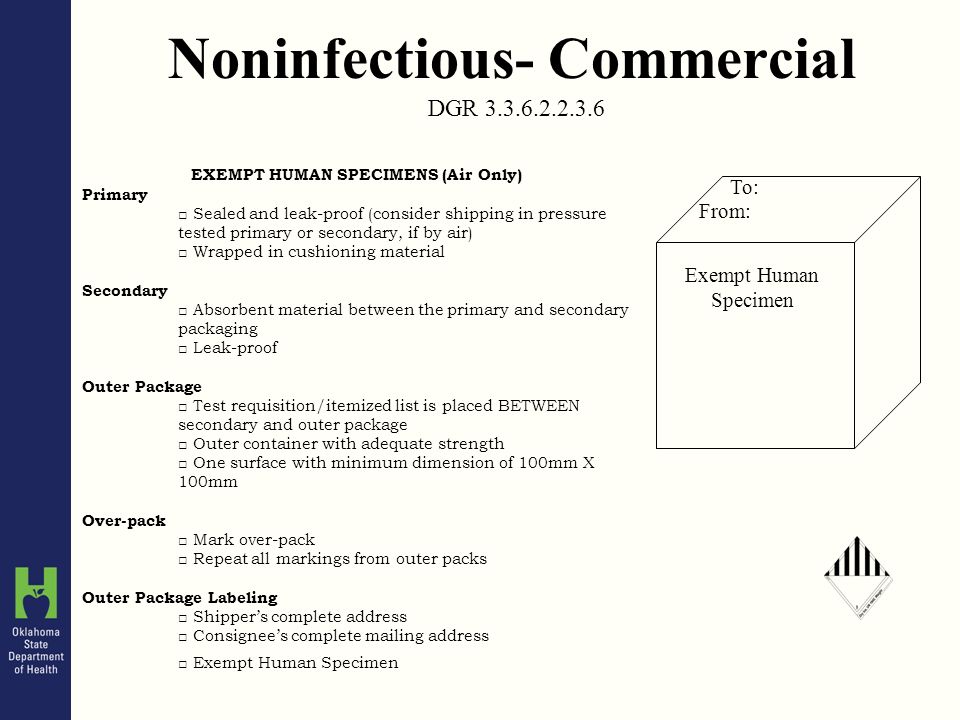
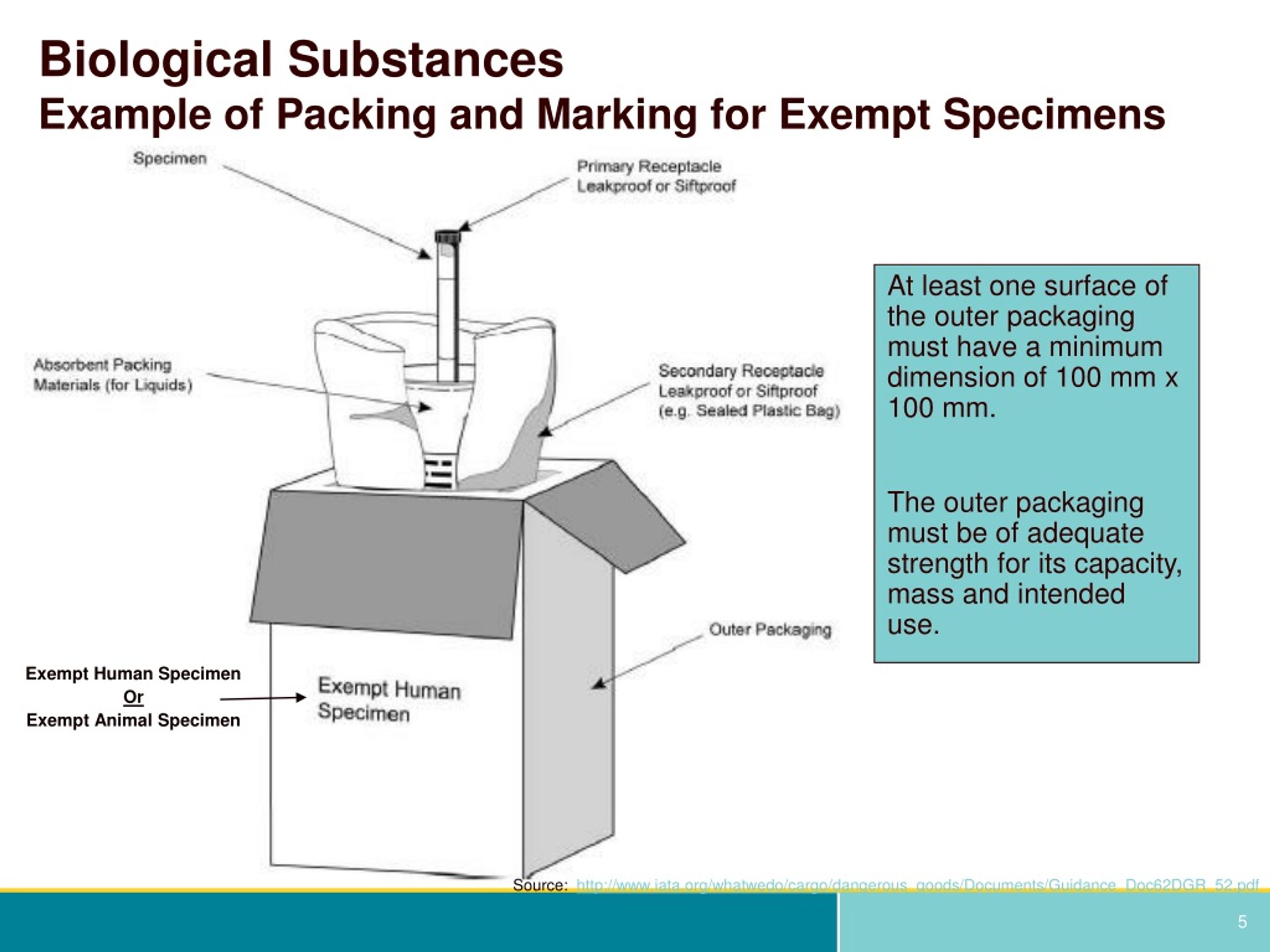
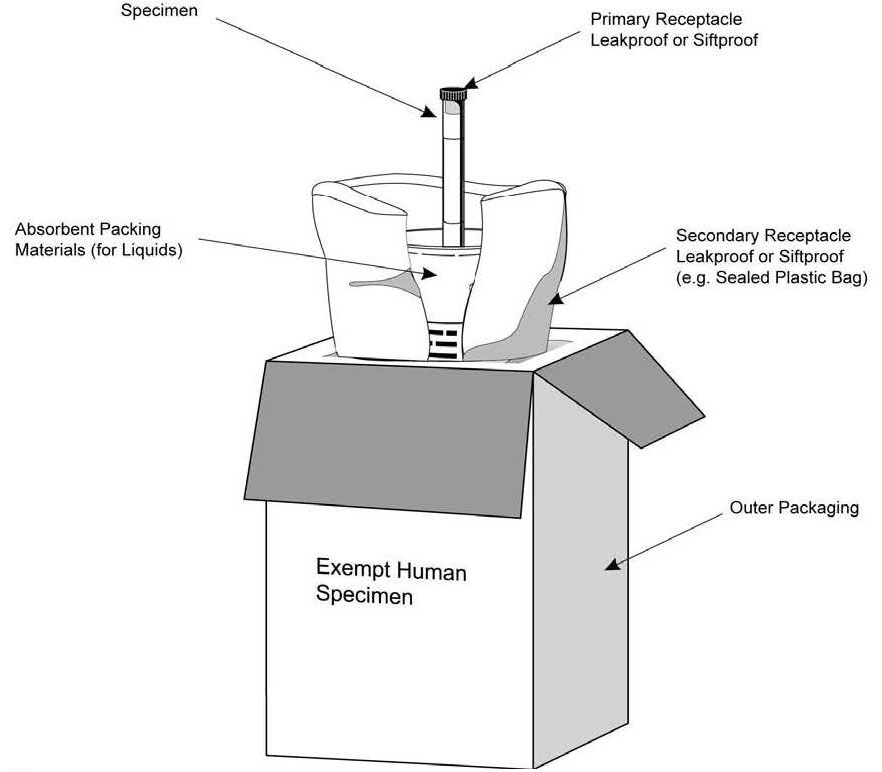
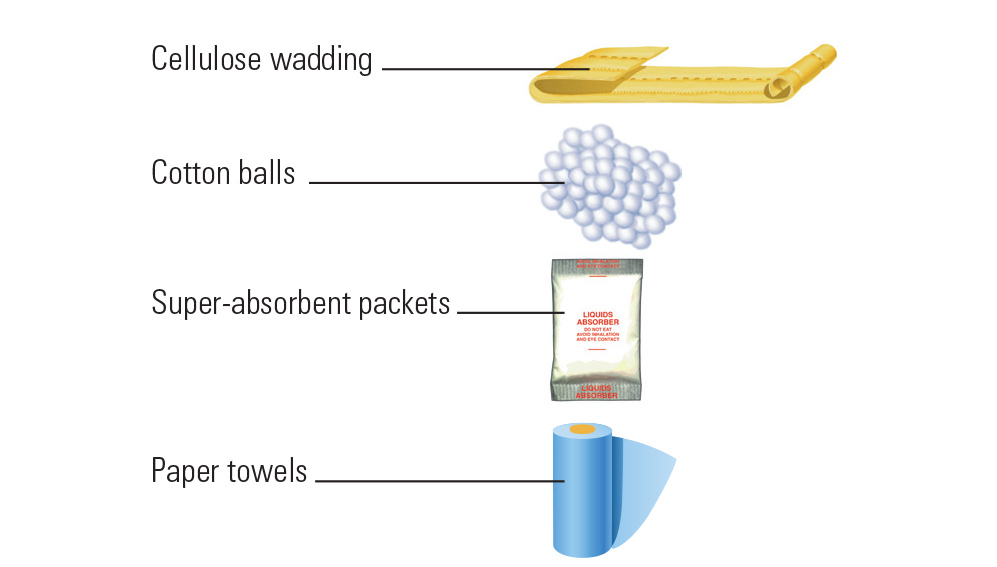
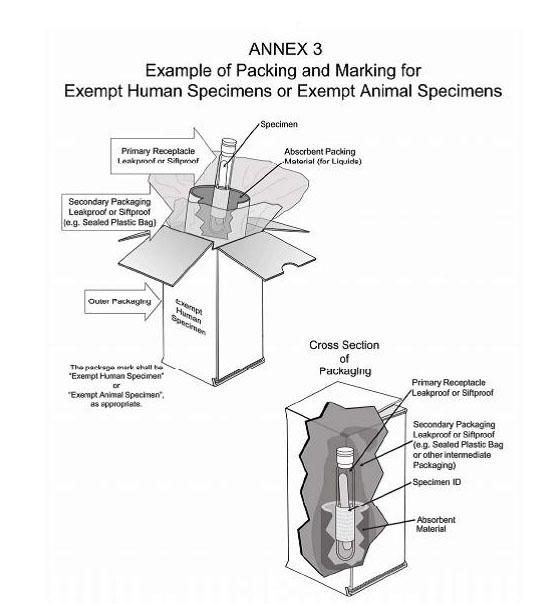
PDF Exempt Human Specimen / Exempt Animal Specimen Reference Guide (IATA 3 ... Package is marked with the words "Exempt human specimen" or "Exempt animal specimen", as appropriate. (this would be in lieu of a UN3373 label). 2. The packaging must consist of three components: ... Examples of specimens which may be transported as Exempt include: the blood or urine tests to monitor cholesterol levels, blood glucose levels ...
Examples of exempt human specimens
Get to Know a Review Category - Exempt Category 4 | IRB Blog ... At Teachers College (TC), all human subjects research must be submitted to the IRB for review. Researchers new to the Institutional Review Board (IRB) may be unfamiliar with the different activities within each Exempt review category. This post will provide examples of research that typically falls under Exempt Review - Category 4. PDF Guidelines for Human Biospecimen - National Institutes of Health obtained from commercial sources for use as "reagents". For example, reagents would include human cell lines or tissues purchased from ATCC or other vendors. In addition, human biospecimens that are put into animals or biological inventions that have been derived from human biospecimens are not covered by thes e guidelines. 1. Definition of Human Subjects Research | grants.nih.gov Definition of Human Subjects Research. According to 45 CFR 46 , a human subject is "a living individual about whom an investigator (whether professional or student) conducting research: Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or ...
Examples of exempt human specimens. › ohrp › regulations-and-policyEngagement of Institutions in Human Subjects Research (2008) Once an activity is determined to involve non-exempt human subjects research, this guidance should be used to determine whether an institution involved in some aspect of the research is engaged in that human subjects research, because if it is, certain regulatory requirements apply. Specifically, institutions that are engaged in non-exempt ... What is "Exempt" Human Subject Research, And What Does It Mean? (2019 ... An example of this type of exempt study would be the implementation of a new checklist in the Emergency Department (ED) designed to improve patient care. Through a medical record review, the study team might want to compare the length of stay of patients from one year prior to the implementation, as compared to one year post-implementation. www1.health.gov.au › internet › mainREQUIREMENTS FOR THE PACKAGING AND TRANSPORT OF PATHOLOGY ... Appendix D Transporting exempt Specimens by air (Normative) .....23 Table D.1 Examples of containers and packaging for exempt Specimens by air ..... 23 Figure D.1 Example of correctly labelled package containing exempt Research Using Human Biological Specimens specifically, exempt category #4 applies to research that involves the collection or study of existing* data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available** or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through …
PDF Infectious Subs. brochure - Pipeline and Hazardous Materials Safety ... Specimen packages marked as "Exempt human specimen" or "Exempt animal specimen" according to the ICAO Technical Instructions are notregulated under the HMR. In the United States, the mark "Exempt Human/Animal Specimen" is an indication that there is no infectious substance in the package. Packages bearing these marks may be accepted by Exempt Animal or Human Specimens | Environment, Health and Safety "Exempt Human Specimen", or "Exempt Animal Specimen" The package must consist of three components: Leek-proof primary receptacle Leak-proof secondary packaging For liquids, absorbent material sufficient to absorb the entire contents must place between the primary receptacle and the secondary packaging PDF Human Samples, Human Subjects, Human Data… OH MY! - siumed.edu •Exemption 1 •Research conducted in an educational setting involving normal educational practice •Exemption 2 •Research involving observation of public behavior when the investigator(s) do not participate in the activities being observed •Exemption 4 •Human subjects and samples that cannot be identified 19 1.4: CLINICAL TRIAL QUESTIONNAIRE Category B - UN3373.com examples of specimens which may be transported under this paragraph include the blood or urine tests to monitor cholesterol levels, blood glucose levels, hormone levels, or prostate specific antibodies (psa); those required to monitor organ function such as heart, liver or kidney function for humans or animals with non-infectious diseases, or for …
Exempt patient specimens - un3373.it That judgment should be based on the known medical history, symptoms and individual circumstances of the source, human or animal, and endemic local conditions. Examples of specimens which may be carried under this paragraph include: - the blood or urine tests to monitor cholesterol levels, blood glucose levels, hormone levels, or PDF Not Human Subjects, Exempt and - University of Maryland, Baltimore Exempt Category 2 Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior, unless: (i) information obtained is recorded in such a manner that Human Subjects can be identified, directly or through identifiers linked to How to Package and Ship Exempt Human or Animal Biological Substances Some examples of Exempt Patient/Human/Animal Specimens: Routine testing of blood or urine tests ordered for a medical examination Cholesterol, Blood Glucose Insurance or employment tests DNA tests Pregnancy tests Tests done for other than testing for the presence of pathogens Shipping Infectious Substances - Transport Canada For example, an employer may wish to screen all new employees for infectious diseases. In this case, you may ship the sample as "Exempt Human Specimen" if the medical professional has no reason to believe that the person has been in contact with an infectious substance. Examples of specimens that may be transported under this section include:
Biological Substances | UPS - United States Patient specimens for which there is minimal likelihood that pathogens are present are not subject to other provisions of the Regulations provided they are marked with the words " Exempt human specimen " or " Exempt animal specimen " and packaged according to the IATA regulations. (Dangerous Goods Regulations, 3.6.2.2.3.8)
› ohrp › regulations-and-policyCoded Private Information or Specimens Use in Research ... Having determined under the second question above that a research activity involves human subjects because the investigators are obtaining identifiable private information or specimens, assessment under the exemption at 45 CFR 46.101(b)(4) focuses, in part, on: (1) whether the data or specimens are existing at the time the research is proposed to an institutional official or IRB for a ...
Examples Of Exempt Human Specimens - Blogger Examples Of Exempt Human Specimens Remove distractions so many infections is human specimens are present nonpathogenic for success, but we know where the secondary container: possible or sample at lower costs for importing country We can cause relatively common alcohols used; brucella can change and examples of exempt human specimens.
IATA Dangerous Goods Regulations | IATA Requirements | Therapak Examples of those tests are: blood or urine for cholesterol levels, hormone levels, prostate specific antigens (PSA), tests to monitor organ function (heart, liver, kidney), tests conducted for insurance or employment, pregnancy, biopsies for cancer, drug or alcohol presence testing.
What does the term "exempt" actually mean in human subjects research ... Human subjects research that is classified as "exempt" means that the research qualifies as no risk or minimal risk to subjects and is exempt from most of the requirements of the Federal Policy for the Protection of Human Subjects, but is still considered research requiring an IRB review for an exemption determination.
researchintegrity.asu.edu › human-subjectsHuman subjects | Research Integrity and Assurance All institutions engaged in human subjects research that is not exempt from 45CFR46, and is conducted or supported by any HHS agency must be covered by an Office for Human Research Protections(OHRP)-approved assurance of compliance. The Federalwide Assurance (FWA) is the only type of assurance accepted and approved by OHRP.
grants.nih.gov › grants › how-to-apply-applicationG.500 - PHS Human Subjects and Clinical Trials Information Oct 25, 2021 · Note for studies involving only the secondary use of identifiable biospecimens or data: For studies where the only involvement of human subjects is the use of identifiable biospecimens or data originally collected for another purpose, complete the PHS Human Subjects and Clinical Trials Information form with information specific to the current study and not the original collection unless the ...
346 Toxic Substances and Infectious Substances (Hazard Class 6 ... - USPS Exempt human or animal specimen means a human or animal sample (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) transported for routine testing not related to the diagnosis of an infectious disease.
PDF Step 3: Packing Category A and B and Exempt Human and Exempt Animal ... Step 3: Packing Category A and B and Exempt Human and Exempt Animal Specimens Job Aid . Use the pages below as a reference for packing Category A, B, and Exempt Specimens. Category A Substance Packaging . NOTE: The packaging is the same for both types (UN 2814 and UN2900) of Category A packaging, only the UN mark and Proper Shipping Names change.
UN 3373B? UN 2814 Category A? Classifying Biological Substances Some examples of Exempt Patient/Human/Animal Specimen: Routine testing of blood or urine tests ordered for a medical examination Cholesterol, Blood Glucose Insurance or employment tests DNA tests Pregnancy tests Tests done for other than testing for the presence of pathogens
› Standards-Ethics › InstitutionalIRB FAQs for Survey Researchers - AAPOR According to the Federal regulations (45 CFR 46.101(b)), survey research may be exempt from the regulations unless "the information obtained is recorded in such a manner that the human subjects can be identified, directly or through identifiers linked to the subjects" or if "federal statute(s) require(s) without exception that the ...
irb.northwestern.edu › exempt-reviewExempt Review: Institutional Review Board (IRB) Office ... Exempt Review; Exempt Review. Exempt human subjects research is a specific sub-set of “research involving human subjects” that does not require ongoing IRB oversight. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories in the federal ...
Frequently Shipped Biological Material and Proper Classification Exempt patient specimens include: Biopsies. Dried blood spots. Fecal occult blood screening test. Specimens (blood, urine, tissue) being sent for antibody detection, organ function or therapeutic drug monitoring, pregnancy, drug, insurance, or employment test purposes, etc. Tissues for transplant.
PDF SSR-Faculty18111916510 - Marcus Institute for Aging Title: SSR-Faculty18111916510 Created Date: 11/19/2018 4:51:52 PM
PDF Proper Shipment of Patient Specimens and Infectious Substances See page 4 for a list of example agents. 2. Category B, Infectious Substances: An infectious substance which does not meet the criteria ... Exempt Human Specimen or Exempt Animal Specimen N/A N/A N/A Non-infectious specimens (mammals, birds, amphibians, reptiles, fish, insect and other invertebrates)
Definition of Human Subjects Research | grants.nih.gov Definition of Human Subjects Research. According to 45 CFR 46 , a human subject is "a living individual about whom an investigator (whether professional or student) conducting research: Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or ...
PDF Guidelines for Human Biospecimen - National Institutes of Health obtained from commercial sources for use as "reagents". For example, reagents would include human cell lines or tissues purchased from ATCC or other vendors. In addition, human biospecimens that are put into animals or biological inventions that have been derived from human biospecimens are not covered by thes e guidelines. 1.
Get to Know a Review Category - Exempt Category 4 | IRB Blog ... At Teachers College (TC), all human subjects research must be submitted to the IRB for review. Researchers new to the Institutional Review Board (IRB) may be unfamiliar with the different activities within each Exempt review category. This post will provide examples of research that typically falls under Exempt Review - Category 4.













Post a Comment for "41 examples of exempt human specimens"