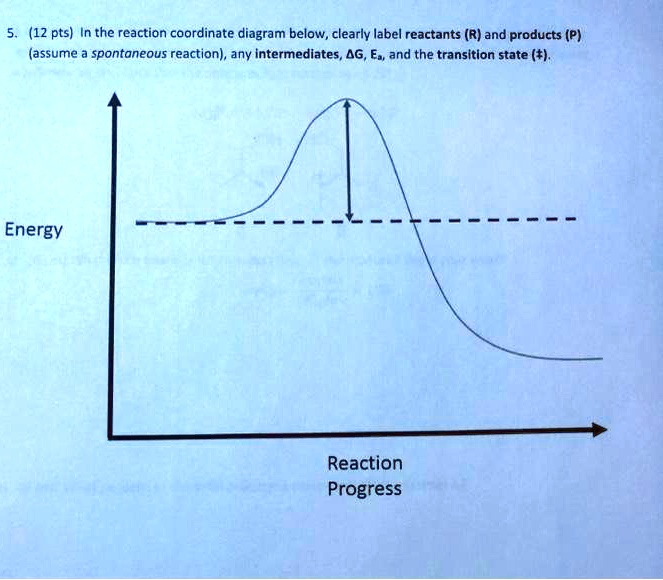
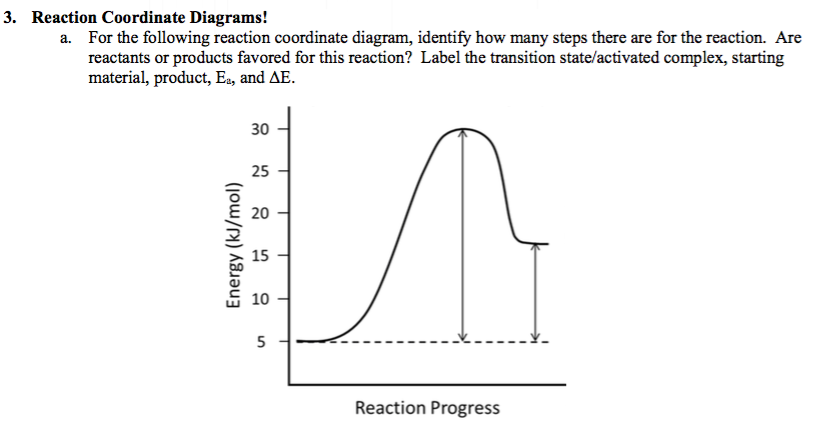
38 label the following reaction coordinate diagram
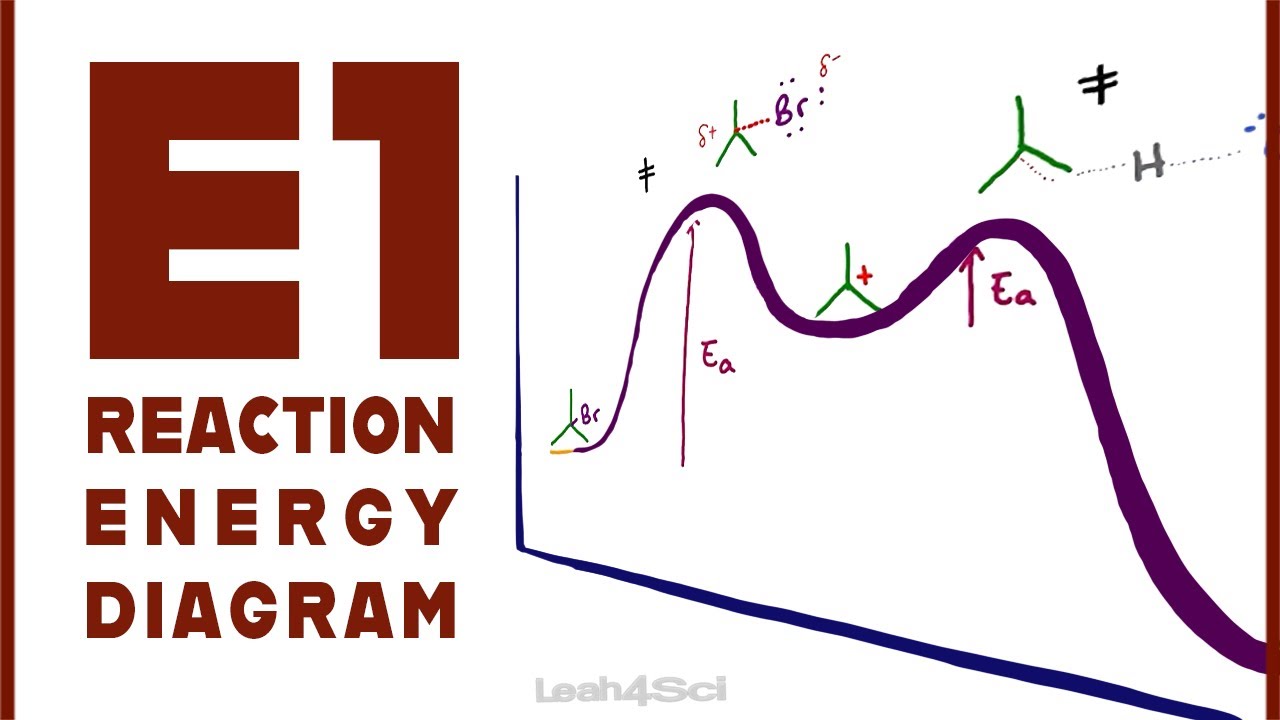
Solved Draw Reaction Coordinate Diagrams For The Following Rea Is diagrams determiningb step the reaction second a is step coordinate high 5 three problem intermediate a chapter 1st endothermic Chemical edition reactionsa f which is the correct reaction coordinate diagram for the following ... Assertion Consider the following two bromides I and II undergoing solvolysis reaction in boilding ethanol : I is less reactive than II in the given so asked Dec 23, 2021 in Chemistry by Anshu Priya ( 24.3k points)
SOLVED: Given the following reaction coordinate diagram Label the ... VIDEO ANSWER:question 99 is really about reading a reaction. Coordinate diagram. The reaction coordinate diagram that is given looks something like this, where the reactant on the left are at a higher energy than the products on the right. We see that there are three transitions, one activation energy to activation energies, three activation energies, three activation energy being the ...

Label the following reaction coordinate diagram
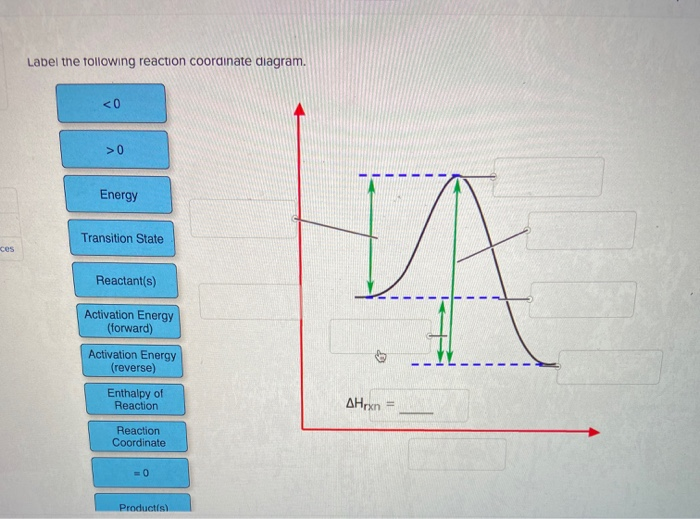
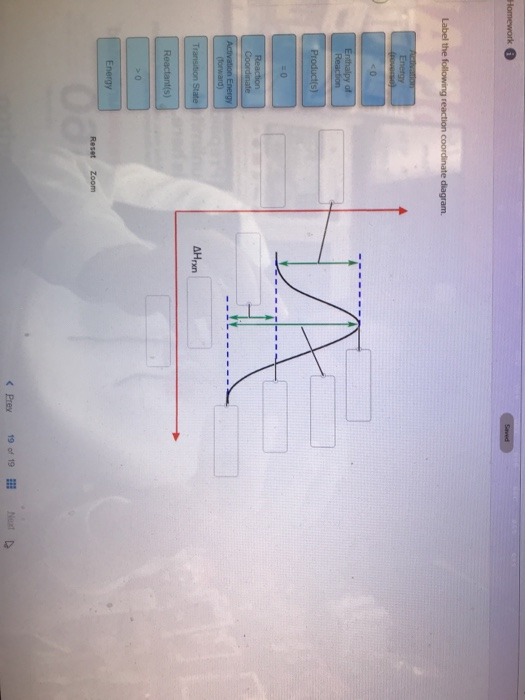
Label The Following Reaction Coordinate Diagram. : A Draw A Reaction ... Draw a reaction coordinate diagram for the following reaction in which c is the most stable and b the least stable of the three species and the transition state . Energy reactant(s) transition state product(s) activation energy (forward) . Label the following reaction coordinate diagram. Is the forward reaction endothermic or exothermic? D) the ... 5.3. Reaction coordinate diagrams - Lumen Learning Energy diagrams for these processes will often plot the enthalpy (H) instead of Free Energy for simplicity.The standard Gibbs Free Energy change for a reaction can be related to the reaction's equilibrium constant (K eq) by a simple equation:ΔG˚ = -RT ln K eq where: K eq = [product] / [reactant] at equilibrium [Wiring Diagram] Reaction Diagram Labeled - osaxen.com Transcribed image text: Label the following reaction coordinate diagram. Energy Reactant(s) Transition State Product(s) Activation Energy (forward). Is the forward reaction endothermic or exothermic? b. Which has the higher potential energy, the reactants or the products? What Is Required? You need to label.
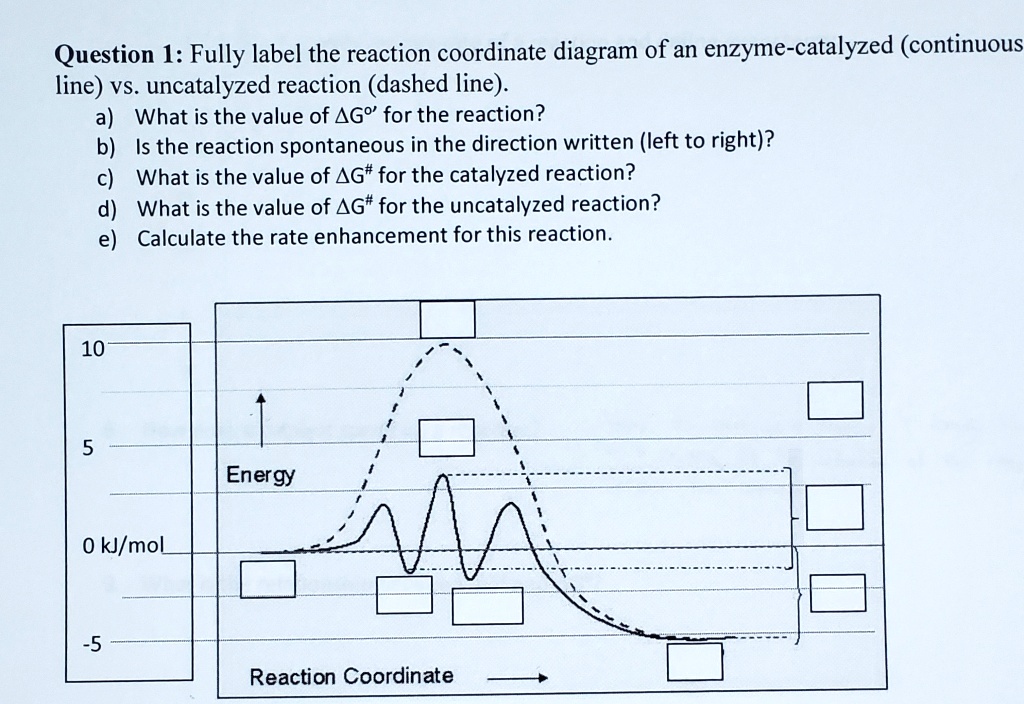
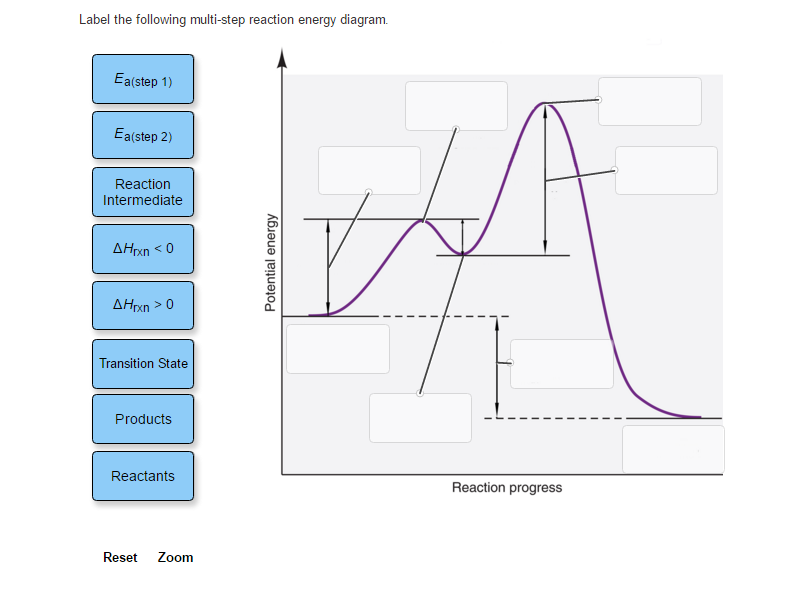
Label the following reaction coordinate diagram. SOLVED:a. Draw and label the reaction coordinate diagram for ... - Numerade this question is in reference to drawing, reading and understanding a reaction. Coordinate in terms of activation, energies in the forward and reverse direction and based on activation energies, the rate at which the reaction occurs in the forward and reverse directions. This is a multi step reaction. There's two steps that are involved. We have a going to be, and then we have be going to see ... Chapter 7 Flashcards | Quizlet On this graph, the x-axis is the reaction coordinate, while the y-axis is energy. Adding a catalyst to a reaction can stabilize the transition state, thereby reducing the activation energy of that reaction. However, the overall free energy of the reaction remains the same with or without a catalyst. Label the following figure. Solved Label the following reaction coordinate diagram. - Chegg Label the following reaction coordinate diagram. Energy Reactant (s) Transition State Product (s) Activation Energy (forward) Transition State Activation Energy (forward) Energy Enthalpy of Enthalpy of Reaction Product (s) Reaction AHrxn Reactant (s) Reaction Coordinate Reaction Coordinate Reset Zoom Which is the correct reaction coordinate diagram for the following ... Correct option is D) Solution:- (D) The given reaction is followed by S N 1 mechanism. The reaction proceeds in two steps - a slow step (high activation energy) followed by a fast step (low activation energy). Thus the (D) is the correct coordinate diagram for the given solvolysis reaction. Solve any question of Chemical Thermodynamics with:-.
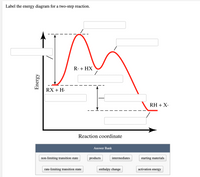
Biochem Chapter 7 Flashcards | Quizlet Label the different energies on the following energy diagram. On this graph, the x-axis is the reaction coordinate, while the y-axis is energy. Adding a catalyst to a reaction can stabilize the transition state, thereby reducing the activation energy of that reaction. Labeling Parts of a Reaction Coordinate Diagram - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Reaction Coordinate Diagram practice problems Quiz - Quizizz What does arrow #3 represent in this reaction coordinate diagram represent? answer choices . activation energy (E a) potential energy of the reactants (PE reactants) potential energy of the products (PE products) change in heat of reaction (ΔH rxn) activation energy (Ea) chem (kinetics pt 2) Flashcards | Quizlet Label the following reaction coordinate diagram by matching between letters and numbers: (diagram in kinetics pt 2 folder in energy diagram folder on desktop) 1- J 2- F 3- A 4- D 5- E 6- B 7- L 8- C 9- K -We can use a 2-D potential energy surface to depict the progress of a reaction.
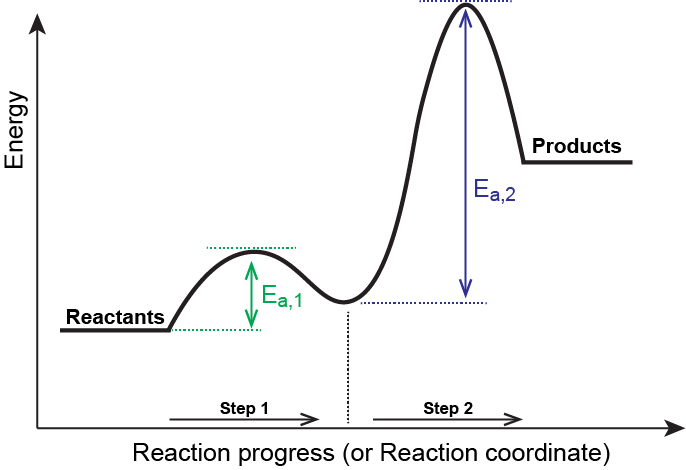
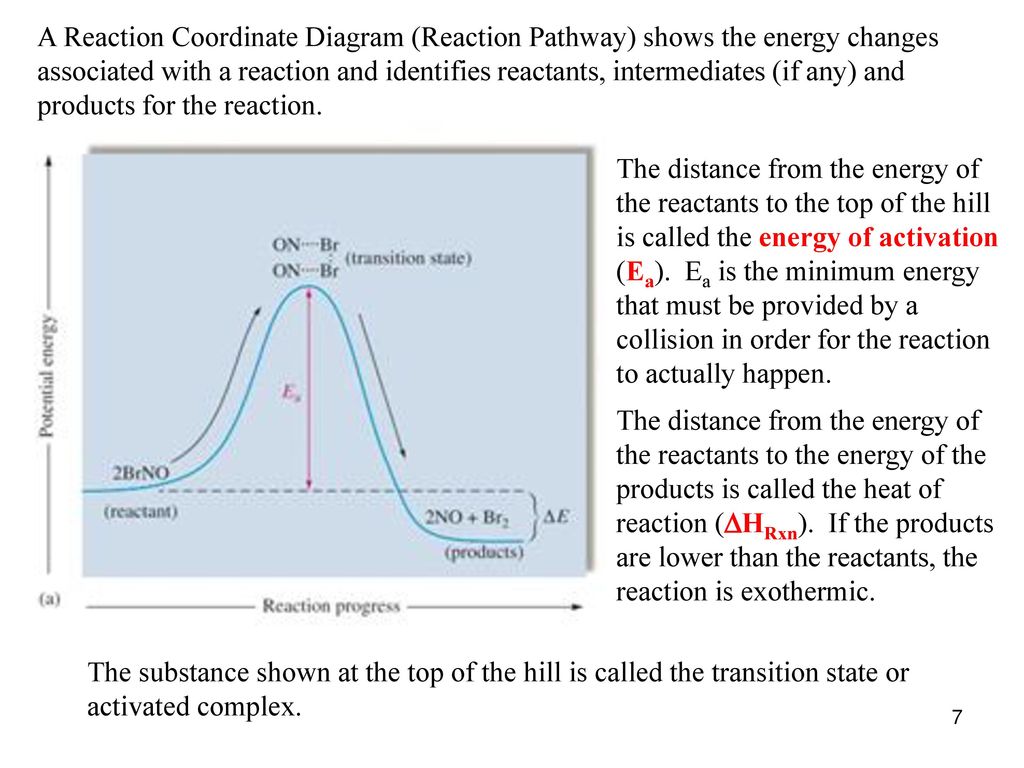
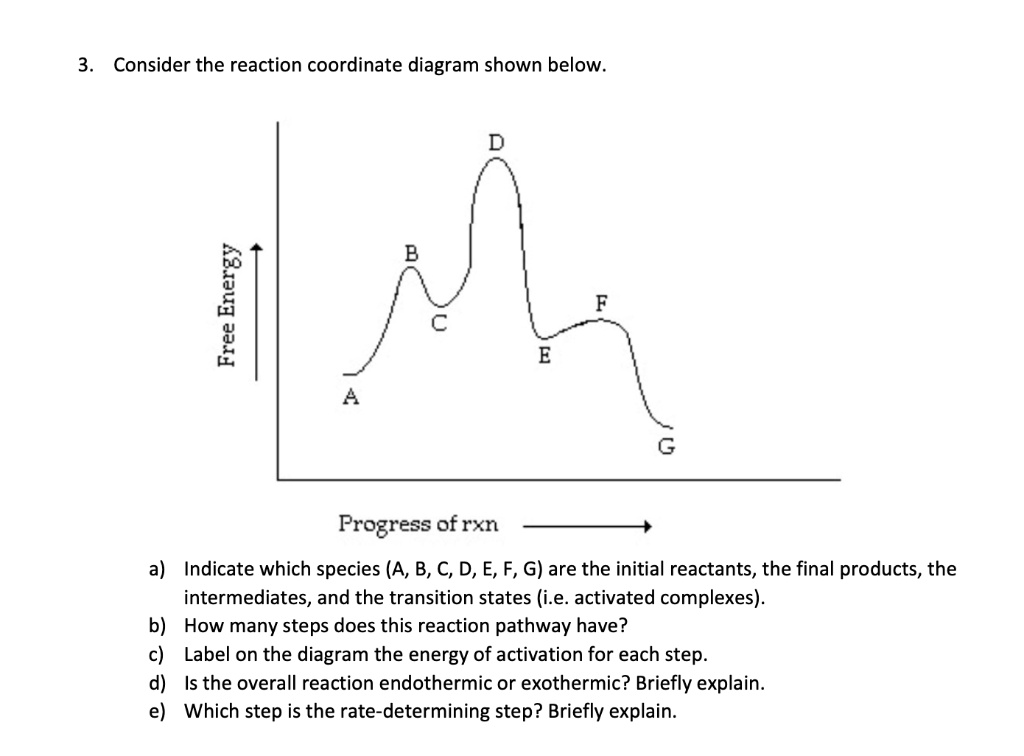
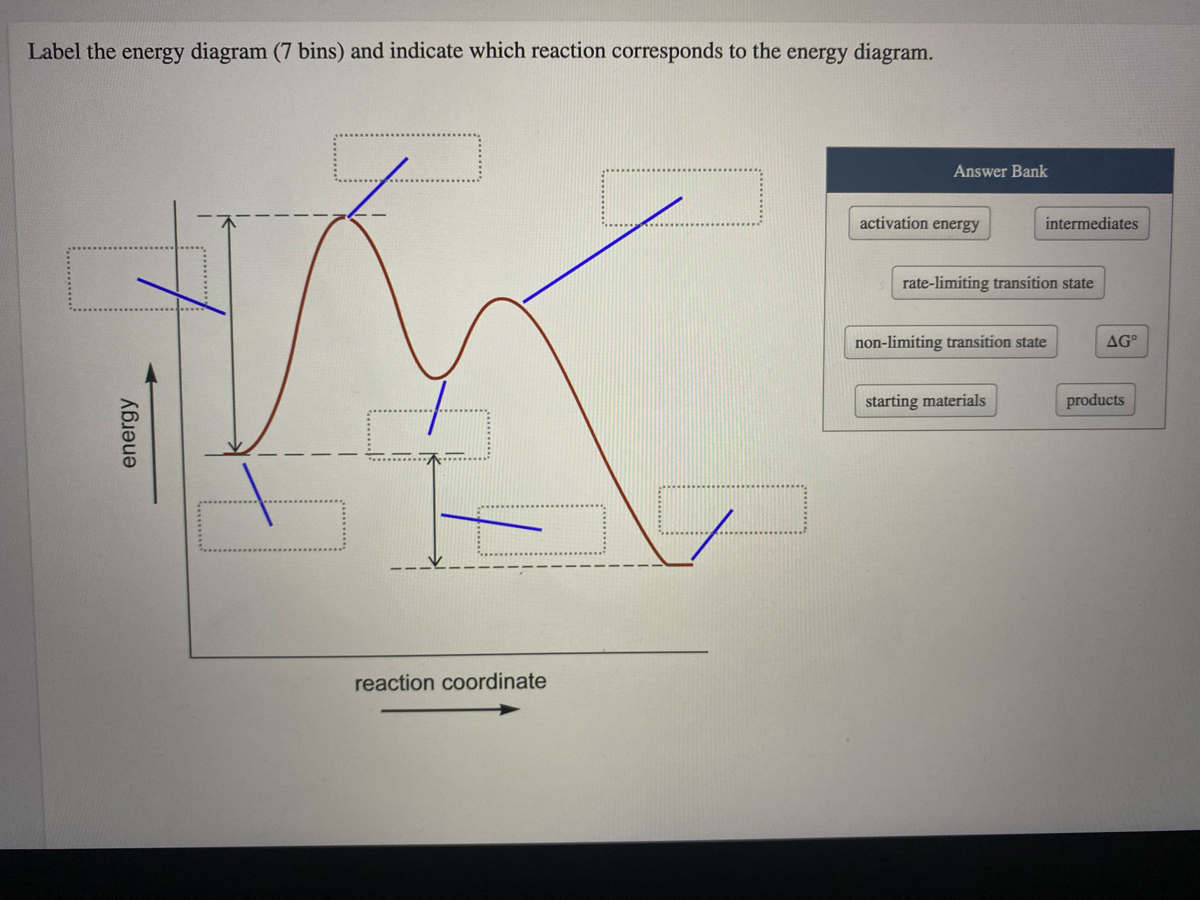
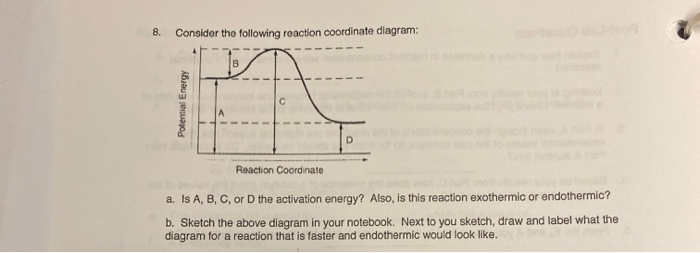
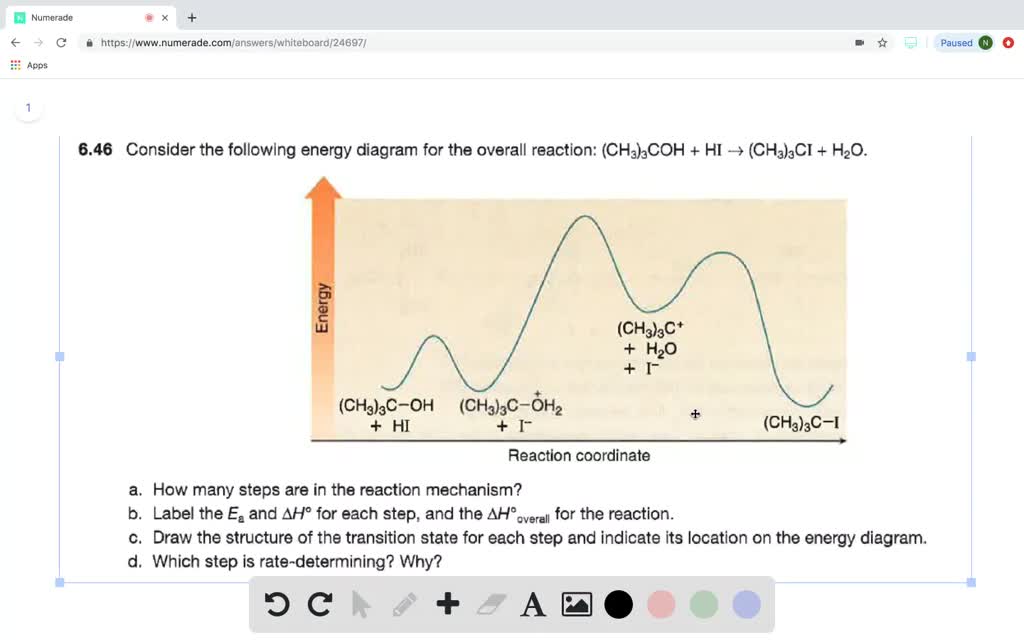
Arrhenius Theory and Reaction Coordinates - Chemistry 302 The reaction above has three steps (three barriers) and two intermediates. On the far left of the diagram are the reactant species and on the far right are the product species. Transition State The transition state is the high energy point between two minima along the reaction coordinate. Each step in a mechanism will have a transition state. The Reaction Coordinate Diagram Questions - Chem Homework Help Draw the complete reaction mechanism and the major product of the reaction. Be sure to indicate stereochemistry in the intermediates and product and label the product as racemic if appropriate. (275 words Label The Following Reaction Coordinate Diagram : Answered 1 Answer The ... Label the following reaction coordinate diagram by matching between letters and numbers: You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. The diagram below is called . Start by drawing and labeling the reaction coordinate diagram. Label The Following Reaction Coordinate Diagram. : Quiz Worksheet ... A) the activation energy is 10. 21.(9 pts) draw the reaction coordinate diagram for the following et reaction label all the following components: Energy Profile Chemistry Wikipedia from upload.wikimedia.org A graph is shown with the label, "reaction coordinate," on the x figure 1.
Reaction coordinate - Wikipedia In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities.In molecular dynamics simulations, a reaction coordinate is called collective variable.. These coordinates can sometimes represent a real coordinate system (such ...
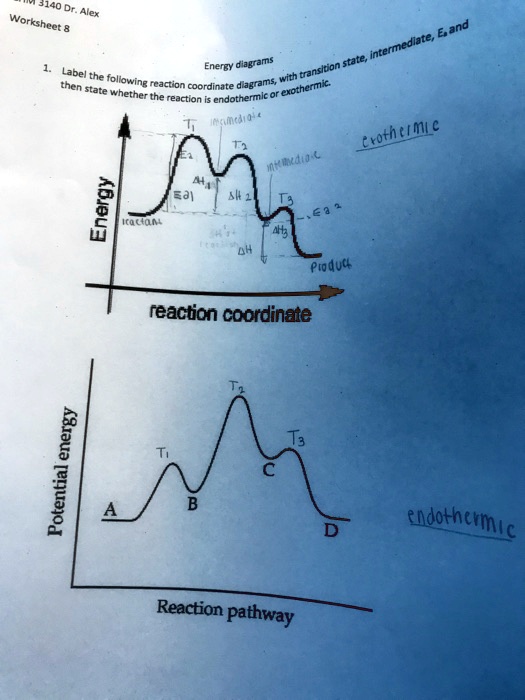
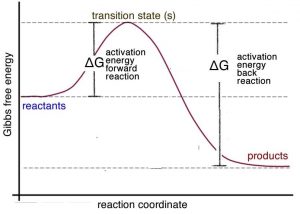
Exam 2 (part 2 - reaction coordinate diagram) Flashcards | Quizlet Reaction Coordinate Diagram a graph that shows how the energy of a reaction changes as the reaction progresses. The most important parts of the energy diagram are the energy levels for the starting materials, transition state (s) and the final product (s). Exothermic -Products will be more stable than reactants. -A negative Delta H in the reaction.
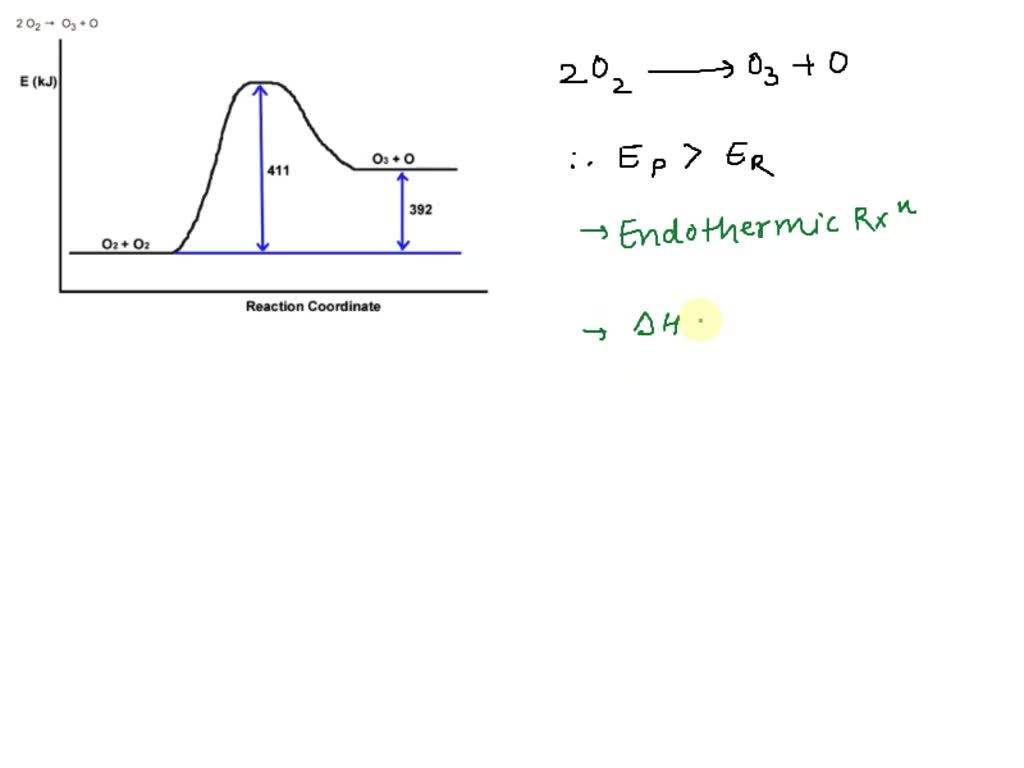
Reaction Coordinate Diagrams - University of Illinois Urbana-Champaign The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction (heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
Label The Following Reaction Coordinate Diagram. - Chapter 1 ... Label the following reaction coordinate diagram by matching between letters and numbers: A graph is shown with the label, "reaction coordinate," on the x figure 1. The diagram below is called . Which has the higher potential energy, the reactants or the products?
Reaction Coordinate Diagrams - College Chemistry - Varsity Tutors The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants. Report an Error
Labelthe following reaction coordinate diagram by matching ... - OneClass Labelthe following reaction coordinate diagram by matching betweenletters and numbers: Answer +20. Watch. 1. answer. 0. watching. 141. views. For unlimited access to Homework Help, a Homework+ subscription is required. Jean Keeling Lv2. 10 Aug 2019. Unlock all answers. Get 1 free homework help answer. Unlock ...
Label The Following Reaction Coordinate Diagram : Solved Integrate The ... This decomposition reaction is consistent with the following mechanism:. Label the following reaction coordinate diagram enthalpy of reaction activation energy (forward) reactant(s) transition state = 0 . Start by drawing and labeling the reaction coordinate diagram. Label the following reaction coordinate diagram.
[Wiring Diagram] Reaction Diagram Labeled - osaxen.com Transcribed image text: Label the following reaction coordinate diagram. Energy Reactant(s) Transition State Product(s) Activation Energy (forward). Is the forward reaction endothermic or exothermic? b. Which has the higher potential energy, the reactants or the products? What Is Required? You need to label.
5.3. Reaction coordinate diagrams - Lumen Learning Energy diagrams for these processes will often plot the enthalpy (H) instead of Free Energy for simplicity.The standard Gibbs Free Energy change for a reaction can be related to the reaction's equilibrium constant (K eq) by a simple equation:ΔG˚ = -RT ln K eq where: K eq = [product] / [reactant] at equilibrium
Label The Following Reaction Coordinate Diagram. : A Draw A Reaction ... Draw a reaction coordinate diagram for the following reaction in which c is the most stable and b the least stable of the three species and the transition state . Energy reactant(s) transition state product(s) activation energy (forward) . Label the following reaction coordinate diagram. Is the forward reaction endothermic or exothermic? D) the ...

















Post a Comment for "38 label the following reaction coordinate diagram"